

Icentia Inc. is a Canadian medical devices and service company that creates innovative medical testing solutions for healthcare institutions. To help improve customer adoption of EART, Icentia asked the research team at MTIC to help them minimize the product learning curve and increase the usage of the tool in a clinical setting.

Funder: Ontario Centre of Innovation
Research Area: Medical Technologies Innovation Centre
Research Team: Rajinder Virk
Future Ready Challenge
In Canada, heart disease is the second leading cause of death after cancer. While heart disease can’t be cured, it can be treated and managed, meaning that effective testing and monitoring for heart disease are crucial to keeping patients healthy.
Long-term continuous electrocardiogram (ECG) monitoring tests are an effective way of measuring the electrical activity of the heart in order to determine if the electrical activity is normal or abnormal. Episodes of atrial fibrillation can be clearly defined in terms of frequency and duration, allowing physicians to optimize patient care. When reviewing long-term continuous monitoring ECG test results, most physicians, cardiologists and ECG technologists use ambulatory ECG reports that have been analyzed by a third-party company - normally the provider of the ECG monitoring device- and perform only the final interpretation of the results. This analysis is often completed using automated detection systems to discover any abnormalities. However, the automated algorithms aren’t always 100% accurate, forcing cardiology technologists to spend most of their time correcting errors in the analysis. To create a more effective workflow that provides better patient care, many healthcare providers are switching to a model that provides healthcare teams with greater control over the reviewing of long-term continuous monitoring ECG test results.
R & D Collaboration
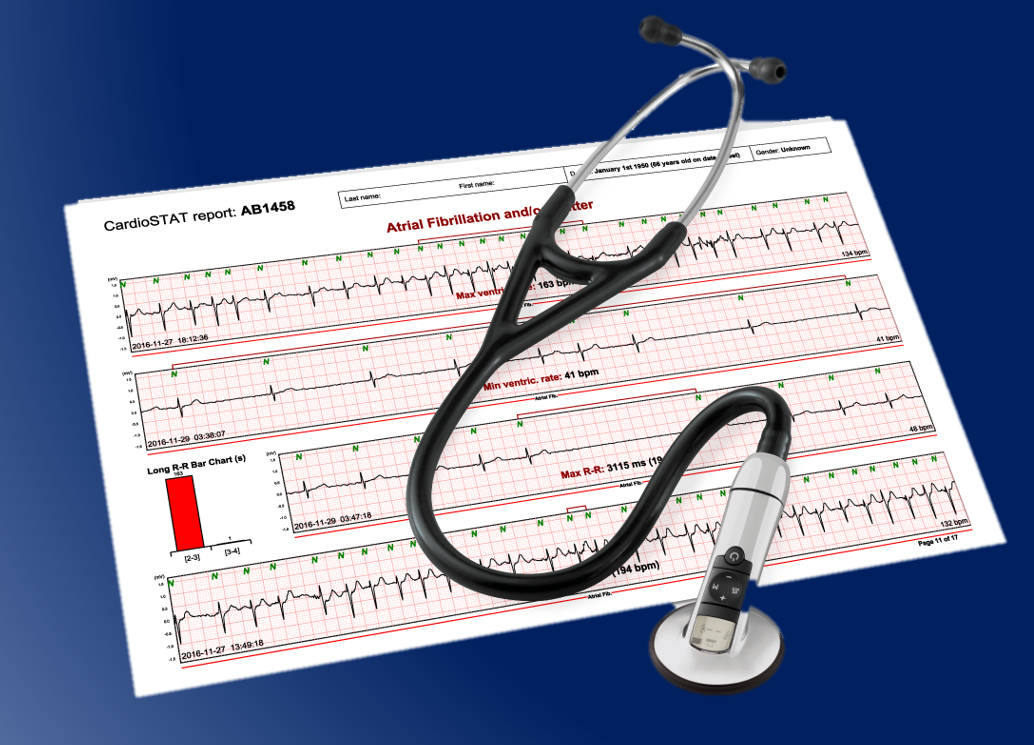
Icentia Inc. is a Canadian medical devices and service company that creates innovative medical testing solutions for healthcare institutions. In addition to developing CardioSTAT, a compact and comfortable portable ECG recorder designed for long-term continuous monitoring, Icentia also created a software program called EART- the ECG Analysis and Reporting Tool- for use by Cardiology technologists.
EART's advanced technology allows technologists to scan through millions of heartbeats collected from patients wearing the CardioSTAT for a time period ranging from 3 to 14 days. It provides and presents data in a way that helps technologists clearly visualize and identify rhythm-based abnormalities. Icentia has found that its customers have been slow to adopt the new tool due to a lack of availability of trained technologists on the EART software. To help improve customer adoption of EART, Icentia asked the research team at MTIC to help them minimize the product learning curve and increase the usage of the tool in a clinical setting.
Innovative Results
To help Icentia reach more customers, the MTIC Research team provided a third-party validation of the product, suggesting product improvements to increase usability and using the data collected to develop educational training tools to assist new users.
A team of students studying in Mohawk’s Cardiovascular Technology program assessed the EART for user-friendliness, providing recommendations for software updates to the product. The research team also surveyed physicians and clinics to collect additional suggestions for product improvement.
“Through its partnership with MTIC, Icentia has not only allowed future medical technicians to train on its latest and most innovative technologies, it has also allowed our engineers to obtain valuable feedback to help improve our products,” says Pierre Paquet, CEO of Icentia Inc. “The results are more efficient solutions and better-trained technicians who are qualified to use them.”

